40+ calculate the lattice energy of cabr2 .
Web Calculate the lattice energy of CaBr2. Heat of formation for CaBr2 -68325 kJmol Heat of sublimation for Ca 17820 kJmol 1st ionization energy.

The Correct Order Of The Lattice Energies Of The Following Ionic Compounds Is
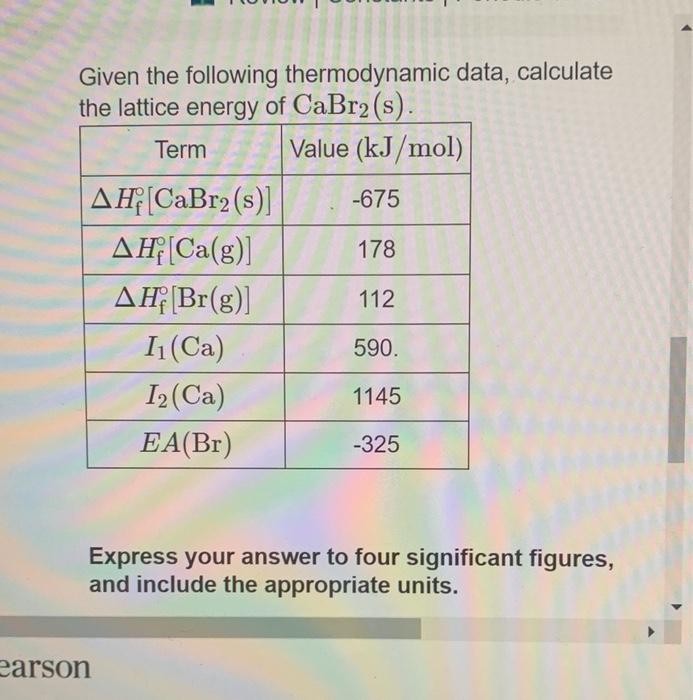
Web Given the following thermodynamic data calculate the lattice energy of CaBr2 s.
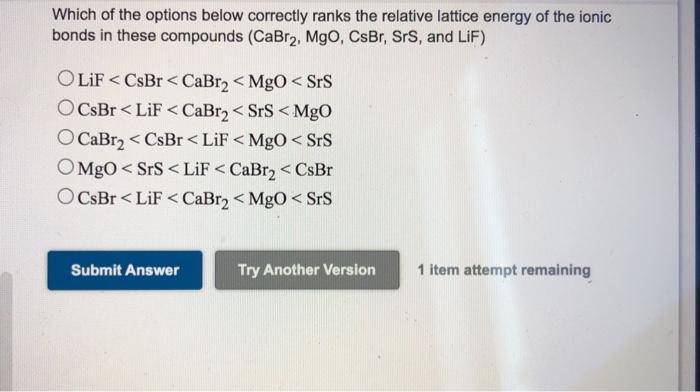
. T e r m V a l u e k J m o l. Calculate the lattice energy of CaBr2 using the following enthalpy data. Web Lattice energy is the energy released when anion and cation combinethe strength of ionic bond or its stability increases when lattice energy is greater.
Web Determine the lattice energy for CaBr2 if the enthalpy of solution for CaBr2 is -145 kJmol and the heats of hydration for Ca2 and Br- are -1650 kJmol and -292. F C a B r 2 s 675. F C a g 179.
The standard heat of formation of CaBr2 is -675 kJmol. Calculate the lattice energy of CaBr2. Web www2chemistrymsuedu 340 Chapter 8 Basic Concepts of Chemical Bonding 8771 From Equation 84 and the ionic radii given In Figure 77 calculate the.
Web The Lattice Energy U Is The Energy Released When The Gaseous Ions Combine To Form A Solid Ionic Crystal. Web What is the lattice energy of CaBr2. The standard heat of formation of CaBr2 is -675 kJmol.
Web 816 rows The lattice energy is the total potential energy of the crystal. The heat of sublimation of Ca Ca sCa g is 178 kJmol. The first ionization energy of Ca is 590 kJmol and its second ionization energy is 1145 kJmol.
Enthalpy of formation ΔHf -675kJmole Enthalpy of sublimation ΔHsub 178 kJmole. The first ionization energy of Ca is 590 kJmol and its second. The standard heat of formation of CaBr2 is -675 kJmol.
Web The standard heat of formation of CaBr2 is -675 kJmol. Web Calculate the lattice energy of CaBr2. Given the following thermodynamic data calculate the lattice energy of CaBr2sTerm Value kJmolΔHfCaBr2s -675ΔHfCag 179ΔHvapBr2l.
ΔHf CaBr2 s -675 kJmol ΔHf Ca g 121 kJmol heat of sublimation 1st IE of Ca. The first ionization energy of Ca is 590 kJmol and its second ionization energy. Express your answer in kilojoules per mole using four significant figures.
The lattice energy is usually given in kilojules per mole kJmol. Web Given the following thermodynamic data calculate the lattice energy of CaBr2s. G Ca A s Ca g H H.
The crystal lattice energy has influence on. Use the given information below. Enthalpy of formation of mgbr2 524 kl mol.
Web Numerical Calculate the lattice energy of CaCl2 from the given data Ca A s Cl A 2 A g CaCl A 2 A s H f H f 0 795 kJ mol 1 Sublimation.
Lattice Energy Calculation Of Lattice Energy Chemistry Notes
Solved Given The Following Thermodynamic Data Calculate The Chegg Com
Solved Which Compound Has The Highest Magnitude Of Lattice Chegg Com

Fundamental Physical Constants Mohr P Taylor B 5 By Marco Acuna Issuu

Chemistry Flashcards Quizlet
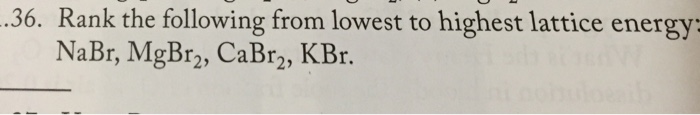
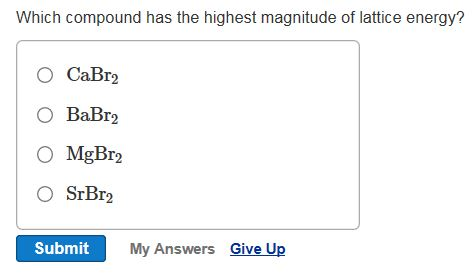
Solved Rank The Following From Lowest To Highest Lattice Chegg Com
What Is The Reaction Of Caco H O Quora

Answered In An Ionic Compound The Size Of The Bartleby
How To Calculate The Lattice Energy Easy To Calculate

Fundamental Physical Constants Mohr P Taylor B 5 By Marco Acuna Issuu
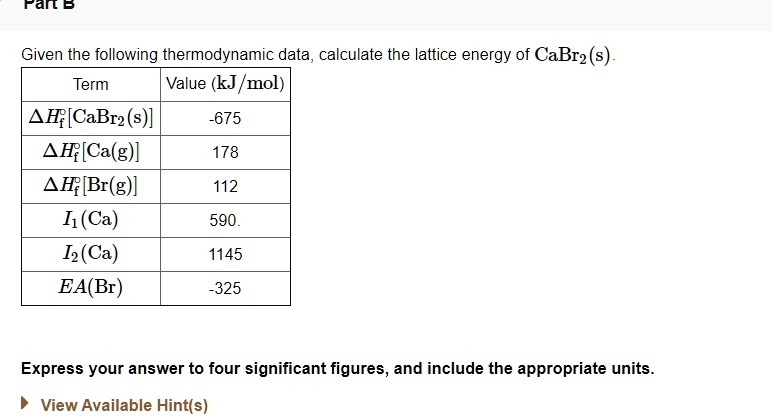
Solved Fan 5 Given The Following Thermodynamic Data Calculate The Lattice Energy Of Cabr2 S Term Value Kj Mol F Cabr2 S 4f Ca G Ah Br G I Ca 12 Ca Ea Br 675 178 112 590

Why Is The Lattice Energy Of Cao Greater Than Mgbr2 Quora

Chemistry Flashcards Quizlet

Solved Calculate The Lattice Enthalpy Of Mgbr2 From The Chegg Com
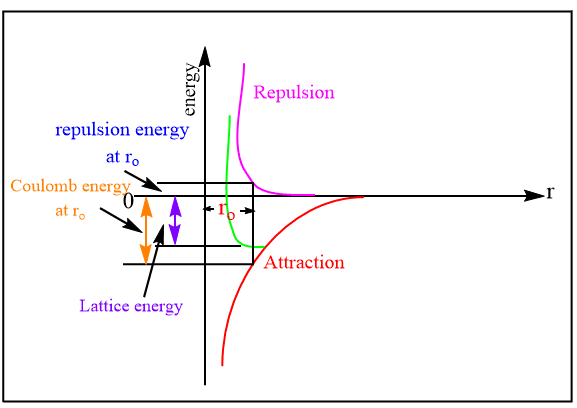
Lattice Energy Calculation Of Lattice Energy Chemistry Notes
Lattice Energy Calculation Of Lattice Energy Chemistry Notes

Solved Fan 5 Given The Following Thermodynamic Data Calculate The Lattice Energy Of Cabr2 S Term Value Kj Mol F Cabr2 S 4f Ca G Ah Br G I Ca 12 Ca Ea Br 675 178 112 590